Abstract
Introduction
Primary myelofibrosis (PMF) and myelodysplastic syndromes (MDS) are clonal hematopoietic neoplasms but have significantly different histopathological findings and clinical courses. MDS median overall survival (OS) ranges from 0.7 to 5.4 years depending on risk stratification; whereas, in PMF median OS ranges from 1.3 to 20 years. According to WHO criteria, certain cytogenetic abnormalities serve as presumptive evidence of MDS even in the absence of morphologic dysplasia namely: -7/del(7q), -5/del(5q), i(17q), -13/del(13q), del(11q), del(12p), del(9q), idic(X)(q13), t(11;16), t(3;21), t(1;3), t(2;11), inv(3), and t(6;9). Many of these are uncommon in PMF. Frequent cytogenetic aberrations identified in PMF include del(13)(q12-22) or der(6)t(1;6). Other anomalies in PMF are del(20q), +8, +9, and +1q. The DIPSS Plus scoring system incorporates some (complex karyotype, +8, -7/7q-, i(17q), -5/5q-, 12p-, inv(3), or 11q23 rearrangements) but not all of aforementioned MDS-related abnormalities. We hypothesized that the presence of MDS-related cytogenetic abnormalities in PMF will be associated with worse prognosis and harbor a unique genomic landscape when compared to cases without such abnormalities.
Methods
This study was approved by the IRB. PMF cases from our institution for whom both NGS data and cytogenetic data were available were identified. NGS panels included Genoptix 21-gene panel, Custom 31 gene TrueSeq Myeloid panel, and standard 54-gene TrueSeq Illumina Myeloid panels (min. allele frequency > 5%). Cytogenetics by karyotyping (performed at LabCorp. Inc) and FISH results (mostly MDS panel, including del(5q), del(7q), del(17p), del(20), and +8) were retrieved from our internal electronic database and recorded. The clinical and pathology reports and/or pathology slides were reviewed to confirm or revise the diagnosis as appropriate which was rendered following the WHO 2008 classification. Fisher's Exact Test and student t-test were used for statistical analysis.
Results
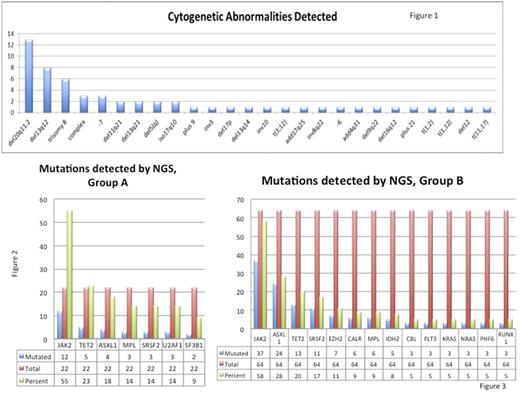
There were 134 patients with a diagnosis of PMF retrieved between 6/2013 and 7/2017. Karyotyping was attempted in 96 cases, of which 10 cases showed no mitotic figures. A total of 86 cases with karyotyping and/or FISH results were included in the study. The average age was 69.7 years. The male to female ratio is 1.4 (male=51; female=37). Myeloid associated gene mutations identified by NGS were seen in 78 of 87 cases (89.7%). Overall, the average number of mutations per case was 2.2/case. Abnormal cytogenetics were found in 49/86 cases (57%) (Figure 1). In the total cohort, 22 (25%) demonstrated MDS related cytogenetic abnormalities (group A) and remaining (64 cases) constituted group B. In group A, the average number of mutations per case was 2. The most commonly mutated genes in group A included JAK2 (12), TET2 (5), ASXL1 (4), MPL (3), SRSF2 (3), U2AF1 (3), and SF3B1 (2) (Figure 2). In group B the average number of mutations per case was 2.3.The most commonly mutated genes in group B were JAK2 (37), ASXL1 (24), TET2 (13), SRSF2 (11), EZH2 (7), CALR (6), MPL (6), and IDH2 (5) (Figure 3). EZH2 and IDH2 were found only in group B and did not occur in group A.
Overall, 6 of 86 PMF patients progressed to AML (7%). AML transformation rate of patients in group A was 6.2% while of patients in group B was 9.5% (p=0.65). Patients with isolated del(13q) (10 cases) were outliers demonstrating longer median OS and thus were segregated for survival analysis. The overall median survival for patients whom data was available was: for group B without del(13q) (n=54) 42.2 months, for patients with del(13) (n=10) 73.7 months, and for group A without del(13q) (n=12) 33.5 months. The difference in OS between group A [without del(13q)] and del(13q) patients was statistically significant (p=0.0533).
Conclusions:
Our study demonstrates that PMF patients with MDS-related cytogenetic abnormalities have a differing genomic landscape when compared with those lacking such abnormalities. Specifically, this group appears to lack EZH2 and IDH2 mutations. Furthermore, we found that PMF patients with MDS related cytogenetic abnormalities (excluding 13q) carried a significantly worse OS when compared to patients with isolated del(13q). These findings may offer further prognostic insights beyond the DIPSS-Plus for patients with PMF. Limited case number warrants a large, multicenter study cohort to validate the findings.
Sallman: Celgene: Research Funding. Padron: Incyte: Honoraria, Research Funding. Komrokji: Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal